Breast Cancer Research
Einstein Shares in $2.9 Million Grant to Combat Breast Cancer Metastasis
May 2, 2017—(BRONX, NY)—Researchers at Albert Einstein College of Medicine and three other institutions are sharing a five-year, $2.9 million grant from the National Cancer Institute (NCI) to study how different types of cells influence breast cancer metastasis—the process by which cancer cells spread from the primary tumor to other parts of the body and that causes 90 percent of breast cancer-related deaths.

John Condeelis, Ph.D. (left) and David Entenberg, M.Sc.Using novel imaging technologies, the principle investigators on the grant—John Condeelis, Ph.D., (Einstein), David Entenberg, M.Sc. (Einstein) and James Castracane, Ph.D. (SUNY Polytechnic Institute)—will identify, quantify and locate the cells that contribute to tumor progression, metastasis and chemotherapy resistance. The researchers will also develop strategies for preventing metastasis from occurring.
Einstein and its institutional collaborators—SUNY Polytechnic Institute, Mount Sinai and the University of Wisconsin-Madison—have developed novel technologies that allow them to directly visualize tumors in real time and experimentally manipulate them in living animals. These technologies combine the microfluidic design expertise of SUNY Polytechnic’s College of Nanoscale Science and Engineering with techniques developed in Einstein’s Gruss Lipper Biophotonics Center that use intravital multiphoton microscopy to visualize tumors through implantable imaging windows. This research partnership has yielded implantable imaging windows containing tiny chambers that can deliver chemical and biological agents to tumor microenvironments and collect cells that migrate from them—all while directly visualizing the tumor’s real-time response over several weeks.
“By merging our institutions’ unique technological strengths, this grant allows us to carry out in animal models the types of studies that were previously possible only in petri dishes,” says Dr. Entenberg, director of technological development within the Biophotonics Center. “This is the most clinically relevant way to study cancer and will allow us to answer questions that just couldn’t be addressed before,”
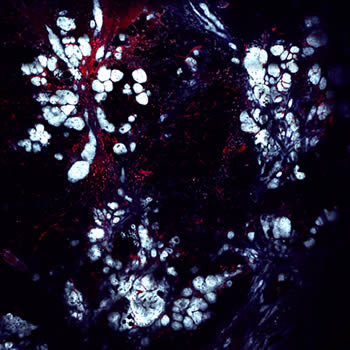
Microscopy techniques developed in the Gruss Lipper Biophotonics Center allow researchers to record real-time images of different types of tumor tissue in living animals. This image is a 3 mm x 3 mm view of breast cancer developing in a living mouse. A fluorescent protein has labeled tumor cells blue. Intravenous injection of a fluorescent dye has stained the blood vessels red. Also visible are macrophages (red dots) that have ingested dye that has leaked from the vessels over time.One of the aims of the grant is to further understand sites in breast tumors called Tumor Microenvironment of Metastasis, or TMEM. Several years ago, Einstein researchers, led by Dr. Condeelis, professor and co-chair of anatomy and structural biology and co-director of the Gruss Lipper Biophotonics Center and the Integrated Imaging Program, discovered that TMEM are sites where breast tumor cells enter blood vessels and spread to other parts of the body.
TMEM form when a specific trio of cells are present together: an endothelial cell (a type of cell that lines blood vessels), a perivascular macrophage(a type of immune cell found near blood vessels) and a tumor cell that produces high levels of Mena, a protein that enhances a cancer cell’s ability to spread. Studies of breast-tumor biopsy tissue show that the presence of high numbers of TMEM is associated with increased risk for metastasis.
“Combining the imaging and microfluidics technologies developed at the collaborating institutions of this grant allow, for the first time, the direct observation of cause and effect relationships between cells in TMEM that are responsible for tumor metastasis and their responses to treatment,” says Dr. Condeelis.
The NCI grant allows the Einstein team to continue its partnership with SUNY Polytechnic Institute researchers,” says Dr. Castracane. “We’re hopeful that our ability to fabricate advanced microdevices will help us and our collaborators learn more about the triggers for breast-cancer metastasis. We’re also pleased to be able to build on ten years of work with our partners in this area of cancer research.”
The grant is titled “Novel Imaging Devices for Measurement and Control of Tumor” (1RO1CA216248-01)
Other Top Stories
9/11 World Trade Center Exposure Linked to Heart Disease Among NYC Firefighters
On Becoming a Physician: New Einstein Students Receive White Coats and Stethoscopes
Novel Therapy for Acute Migraine Shows Promise in Phase 3 Clinical Trial
First Complete Wiring Diagram of an Animal's Nervous System
Multimillion Dollar NIH Grant to Help Reduce Opioid Use & Get Care to People Who Need It
NIH Grant Funds $23 Million Study of Diseases Affecting People Living with HIV
New TAILORx Data Guides Adjuvant Therapy in Younger Breast Cancer Patients
Einstein Celebrates Its 61st Commencement
Bolstering Biopsies: Testing Patients' Individual Cells to Guide Treatment



Tablet Blog