Chaperone-mediated Autophagy
Chaperone-mediated autophagy (CMA) is responsible for the degradation of aproximately 30% of cytosolic proteins in tissues such as liver, kidney and in many types of cultured cells .
This lysosomal mechanism of protein degradation is mainly activated in conditions of stress such as nutrient deprivation or exposure to different toxin compounds. Under those conditions, the substrate proteins for this pathway, all those containing a motif biochemically related to the pentapetide KFERQ , are selectively recognized by a cytosolic chaperone, the heat shock cognate protein of 70 kDa (hsc70) [1]. The interaction between the chaperone and the substrate in the cytosol targets the complex to the lysosomal membrane where it binds to the lysosome associated membrane protein type 2A (LAMP-2A), that acts as a receptor for this pathway [2]. A second chaperone, the lysosomal hsc73 (lys-hsc70), is required for the complete translocation of the substrate protein into the lysosomal matrix [3] where it is completely degraded by the lysosomal proteases [4].
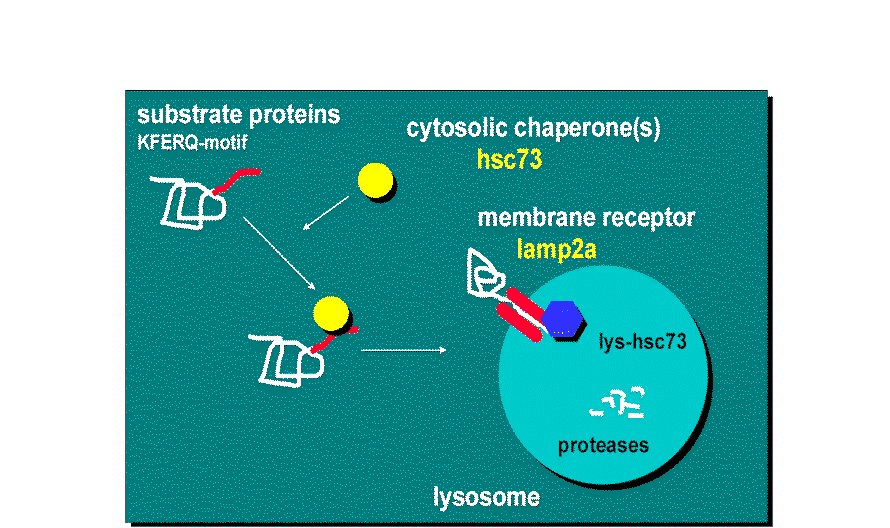
Hypothetical Model of
Chaperone-Mediated Autophagy
.
Substrate proteins for CMA constitute a heterogeneous group of proteins that include among others some glycolitic enzymes, transcriptions factors or its inhibitors, subunits of proteases, calcium-binding proteins and cytosolic forms of secretory proteins.
The transport of substrates for its degradation takes place in molecule by molecule bases and depend on the levels of LAMP-2A in the lysosomal membrane .
Our main goal is to characterize the different components involved in chaperone-mediated autophagy and to identify new players for this pathway.We use an animal model that requires the isolation of intact lysosomes from several tissues (liver, kidney and spleen) of mice and rats. Using an in-vitro system we are able to separately analyze each step involved in the degradation of substrate proteins: binding to the lysosomal membrane, uptake into the lysosome, and complete degradation once at the lysosomal matrix. For the identification of the new components of the systems we are currently using different immunochemical approaches and the yeast two-hybrid system. We are currently tracking down two proteases, coimmunoprecipitated with the lysosomal receptor, that modulate chaperone-mediated autophagy activity by regulating the levels of the receptor.