Autophagy Dysfunction and Immunosenescence
Introduction
╗ Goal
╗ Specific
Aims
Subprojects
╗ Autophagy and T cell activation
╗ Autophagy and T cell
tolerance
╗ Mechanisms of Immunosenescence
Members
Fernando Macian
Rut Valdor Vanessa Hubbard
Ajana
Jordan
Ian Baina
Dr. Fernando Macian
Project
3
will investigate
the role that autophagy plays in the control of T cell responses and
determine if the decrease in autophagic activity with age isinvolved in
the immunosenescent phenotype.
control of T cell responses and
determine if the decrease in autophagic activity with age isinvolved in
the immunosenescent phenotype.
Cellular
homeostasis requires a careful balance between protein synthesis and protein
degradation. Constant protein turnover is necessary not only to reduce
the accumulation of damaged proteins in the cell, and to recycle amino
acids for new protein synthesis, but it also allows for the modification
of protein levels in response to extracellular signals. A major pathway
involved in the degradation of long-lived proteins is autophagy, a
catabolic process that delivers cytoplasmic material to the lysosomes
for degradation. Three main types of autophagy have been described in
mammalian cells: macroautophagy (MA), chaperone mediated
autophagy (CMA) and microautophagy. Different mechanisms control the
regulation of these three forms of autophagy and the targeting of their
substrates to the lysosomes. These systems are critical for proper cell
function, as their failure, as seen in several diseases, leads to the
intracellular accumulation of abnormal proteins, defective regulation of
many cellular processes and altered responses to stress.
Although the
role of autophagy in many tissues and systems has been characterized, it
is still not completely understood whether autophagy has a role
in the regulation of the adaptive immune system and the processes
regulated by autophagy and the mechanism that regulate of MA and CMA
activity in T cells remain to be characterized.
The activity
of MA and CMA has been shown to decrease with age. Our group
has gathered evidence that indicates that aged CD4+ T cells cannot
properly regulate autophagic activity in response to TCR engagement. We
intend, thus, to characterize the functional consequences of this defect
for T cell function in old organisms.
As
with many other systems, the immune system in also affected by age. Immunosenescence
is characterized by a decrease ability of immune cells to mount a
productive response upon exposure to new antigens, accounting for
the increased susceptibility of the elderly to infectious disease and for
the poor responses to vaccination. Alterations of immune responses
with age reflect defects in several cell types, although CD8+ and CD4+ T
cells appear to be particularly affected
. Although the total numbers
of T cells do not seem to change in the elderly, there are clear
differences in the T cell compartment between young and old individuals.
These changes are a consequence of a diminished thymic output, due to the
age-associated thymic involution, increased homeostatic division
, life-long exposure to
pathogens and, likely, aging of the bone marrow-derived T cell precursors.
Old na´ve and memory T cells, and even recent thymic emigrants in aged
mice, respond poorly to antigen and show defects in many signaling
pathways.Paradoxically, the risk of suffering several autoimmune diseases
increases also with age. Peripheral T cell tolerance is maintained mainly
through three different mechanisms: T
cell anergy, regulatory T cells (Tregs) and peripheral
deletion of self-reactive T cells. T cell anergy can be defined as a
long-lasting status of unresponsiveness induced by suboptimal stimulation.
Classical clonal anergy has been described following the two signal model
as a consequence of T cells being stimulated through the TCR (signal 1)
without engagement of costimulatory molecules (e.g. CD28, signal 2),
as opposed to full T cell activation achieved when both signals are
engaged. We have recently demonstrated that signaling through the TCR in
the absence of costimulation results in an unbalanced activation of the
Ca2+/Calcineurin pathway that induces NFAT dephosphorylation and
activation in the absence of AP-1 activation. As a result of this
unbalanced activation, an anergy-specific set of genes is expressed, which
encode proteins necessary for the induction and likely the maintenance of
the anergic status. Signals transmitted through the TCR are able to
implement, thus, two opposite programs: activation and anergy.
Age-associated defects in TCR-induced signaling may, thus, affect not only
T cell activation but also T cell anergy.
Our
goal is to elucidate the role
that autophagy plays in the control of Th cell responses and determine if
the decrease in autophagic activity with age is involved in the
immunosenescent phenotype, characterized by decreased responses to foreign
antigens and increased risk of autoimmunity.
Gaining new insights on the causes that lead to altered T cell function in the elderly, may not only offer new knowledge on the altered regulation of T cell activation and T cell tolerance with age but it may also set the basis for future therapeutic interventions.
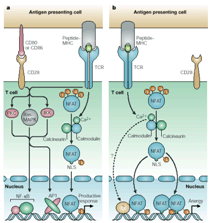
Role of NFAT proteins in the regulation of T cell activation and T cell tolerance

Nuclear trasnlocation of NFAT in
response to calcium signals in T cells
Autophagosome formation in activated T cells