Core A
The administrative core leads, supports and oversees the infrastructure to the CMCR Programs and Cores in order to carry out the research mission. There will be an internal and external committee to govern the CMCR strategic plan and participants include faculty, directors, senior scientists, administrators and administrative staff.
This core will manage:
- grants and fiscal reporting
- internal and external committee meetings, events and seminars
- IACUC approval
- collaboration with external institutions and reporting to NIAID
- Central Interface between the CMCR and other Einstein-Montefiore Departments
- Communication of CMCR’s Scientific Findings and Updates
- CMCR Website
Core B (Pilot Project)
The CMCR will fund innovative pilot projects related to programmatic areas that are part of its mission. A mission of the Pilot Projects Program is to create a funding mechanism, which promotes innovative research in the field of radiation protection and mitigation. In particular, the objective is to provide a mechanism for promising investigators to acquire preliminary data and gather sufficient proof-of-principle to become competitive for subsequent extramural funding in the field of radiation countermeasures. To be considered for Pilot Project funding, proposed projects must be significantly distinct but no substantial overlap with the existing projects of this application.
The CMCR Project Review Committee will select priority areas of study which will:
- help elucidate cellular and molecular mechanisms resulting in radiation mitigation/treatment;
- develop techniques to screen for radiation mitigators;
- predict radiation-toxicity risk and genotoxicity;
- help explain inflammation as a result of radiation injury.
This program also will support rapid implementation of new ideas that may be important for clinical applications of novel technologies and approaches. The Pilot Studies Program in the CMCR will offer outstanding substrates for building on and creating synergy with ongoing research at the participating institutions, as well as for establishing an academic environment and incentives to promote interest in all aspects of investigation in countermeasures.
Core B Application Process
All applicants for pilot projects will be required to electronically submit a one-page letter.
This letter should provide:
- a statement of the project’s aims and hypotheses
- a brief description of the study design
- contact information (including the principal investigator’s title, institutional affiliation, address, telephone number, and e-mail address)
Following submission of the letter of intent, a member of the Project Review Committee or Executive Committee will contact the applicant to provide guidance on proposal development and to suggest consultations with one or more of CMCRs members as needed. This process will ensure that all applicants get advice and direction regarding the development of their research programs, irrespective of the eventual funding decision.
A full NIH-style proposal will be required. The submitted proposal should be single-spaced, with one-inch margins, and its main body (sections 4–6) should not exceed 10 pages. The proposal should consist of the following components:
- Cover letter (1 page)
This should include the project’s title, the principal investigator’s affiliation and contact information and the total budget request.
- Title page, including:
- the project’s title
- the name and affiliation of the principal investigator
- the total budget request
- signatures of the principal investigator and of his/her department chair
- Abstract (150 words maximum)
The abstract should summarize the entire body of the proposal (sections 4–6).
- Specific Aims (1 page maximum)
This section should include a statement of the study aims and hypotheses and a very brief synopsis of the study design and generally follow the NIH format.
- Background and Significance (2 pages maximum)
This section should include a relevant summary of the literature and a specific statement on where the proposed research fits.
- Study Design (6 pages maximum)
This section should include a description of the proposed experimental or study design, planned data analysis (including sample size justification, if applicable), and a timeline of the proposed research activities. A brief discussion of strengths and limitations may also be included.
- Timeline and Budget, with brief justification
The budget should be used in direct support of research-related activities. Funds are restricted to research expenses and generally cannot be used to support travel, faculty salary, or equipment purchases. The total project duration should not exceed 24 months and its total budget should not exceed $75,000/year.
- Personnel List, with curriculum vitae.
Core C (Histopathology Core)
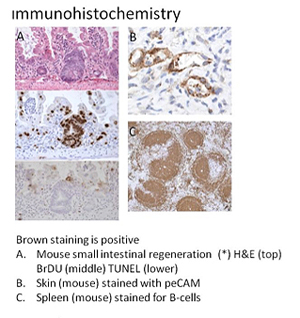 The Histology and Comparative Pathology Facility provides comprehensive histology and histopathology support to Einstein investigators and the Institute for Animal Studies. The facility performs most aspects of tissue evaluation, from necropsy to the final histological diagnostic evaluation. We support quality translational research through an understanding of animal physiology and histopathology, combined with a close working relationship with researchers. The goal of the facility is to have a high-quality histology service as well as a resource for understanding and translating
The Histology and Comparative Pathology Facility provides comprehensive histology and histopathology support to Einstein investigators and the Institute for Animal Studies. The facility performs most aspects of tissue evaluation, from necropsy to the final histological diagnostic evaluation. We support quality translational research through an understanding of animal physiology and histopathology, combined with a close working relationship with researchers. The goal of the facility is to have a high-quality histology service as well as a resource for understanding and translating
in vivo data.
Services:
- Immunohistochemistry & enzyme histochemistry
- Tissue microarrays
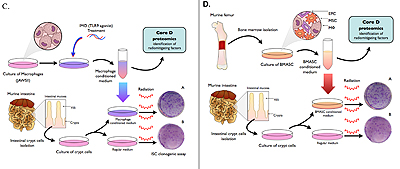
Core D (The Discovery and Target Validation Proteomics Core)
The primary aim of this CMCR Core is to provide a comprehensive characterization of biomarkers indicative of radiation injury, and identification of therapeutic targets using cutting-edge proteomic techniques for all investigators of the Einstein's CMCR.
The Discovery and Target Validation Proteomics Core offers standardized and robust sample preparation for investigators to streamline the protein analysis performed in this facility. Direct consultation to investigators will be offered to address questions of sample preparation and experimental set up for proteomic analysis. The facility offers two different protein identification and characterization platforms: 1D/2D gel electrophoresis and mass spectrometry-based shotgun proteomics. Shotgun proteomics technology has been developed to directly analyze complex protein mixtures in solution using the multi-dimensional protein identification technology (MudPIT).
 By coupling the high-pressure liquid chromatography (HPLC) and tandem mass spectrometry, MudPIT-based analysis provides the sequence and quantification of thousands of proteins. Both labeling (SILAC, iTRAQ, TMT, etc.) and non-labeling methods are available for global protein quantification.
By coupling the high-pressure liquid chromatography (HPLC) and tandem mass spectrometry, MudPIT-based analysis provides the sequence and quantification of thousands of proteins. Both labeling (SILAC, iTRAQ, TMT, etc.) and non-labeling methods are available for global protein quantification.
The proteomics core facility has cutting-edge mass spectrometers such as Linear Ion Trap (LTQ), TSQ Quantum, and LTQXL-Orbitrap with a new fragmentation source, ETD (which preserves labile modifications). High-resolution instruments such as the LTQXL-Orbitrap can provide accurate protein identification and identify protein modifications that are normally hard to detect. In addition, the facility has a computer cluster equipped with proprietary software necessary for protein identification (SEQUEST, MASCOT, InspecT, ProLucid), quantification (CenSus), PTM characterization (ProLucid and InspecT), and data mining. For investigators searching for signaling pathways and global proteome signatures, the proteomics core facility offers web-based data mining software such as the Ingenuity Systems to assist with data analysis.
As a new facility, the Discovery and Target Validation Proteomics Core will make innovative proteomics technology available to collaborating researchers and research groups. The core will coordinate closely with the director, researchers of individual projects, and other cores at the Einstein CMCR. Extensive knowledge from the directors of the Discovery and Target Validation Proteomics Core will support investigations on the mitigation of acute-radiation syndrome by stem cell-based therapies.
Core E
The Einstein Stable Isotope & Metabolomics Core is within the NIH funded Diabetes Research Center at Einstein and anchors the CMCR Metabolomics Core. The Core uses stable isotope flux and metabolite profiling to help formulate and test hypotheses about the metabolic consequences of various changes in gene expression and protein function, in order to guide further integrative systems biology analyses of the underlying mechanisms in diabetes, insulin resistance, obesity, and diabetic complications. The Core objectives includes assessments of plasma, urine and tissue metabolite profiles for diagnosis/characterization of physiological and pathophysiological states. These include key metabolites in the glycolytic, gluconeogenic, pentose phosphate, and tricarboxylic cycle pathways, fatty acid oxidation and complex lipids, such as ceramides, diacylgylcerols, triacylglycerols (TAGs), and phospholipids. In the CMCR framework, the Core will use these methodologies to explore the specific effects that radiation has on the metabolic network of each organ, and to explore the metabolic consequences of protein modification, by radiation, on the function of the metabolic network.
View Powerpoint